| Overview |
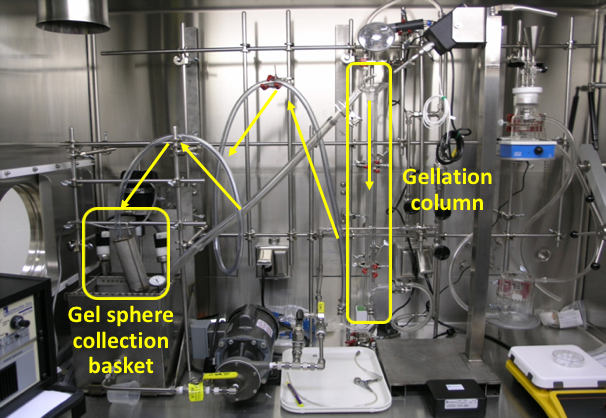
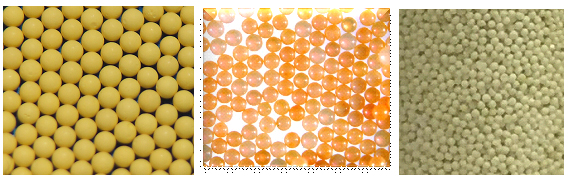
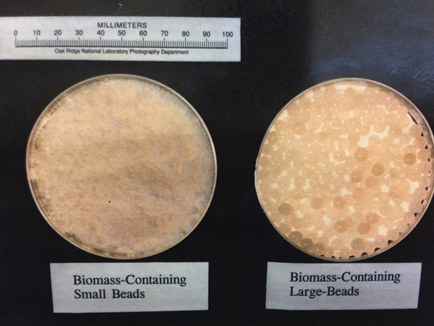
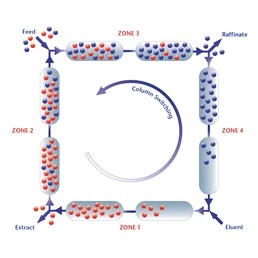
ORNL has the unique capability in synthesis production of inorganic sorbent beads via internal and external gelation processes. The inorganic beads can be made to be nanoporous or mesoporous oxides so that large specific surface areas can be achieved. The composition of inorganic beads ranges from oxides, carbides, sulfides, and their mix. We can molecularly functionalize the surfaces and inner pore surfaces of the inorganic beads. Many other active ingredients (in the powder or molecular form) can be also dispersed, encapsulated, immobilized, or embedded inside the platform inorganic beads. These uniform sized spherical beads (typically 0.1-10 mm in diameter) can be easily packed into a fixed bed for process characterization (adsorptive separations and regenerations). The inorganic sorbents are mechanically strong and particularly good to tolerate high-temperature thermochemical processing/separation conditions.Secondly, ORNL has the hands-on experience in synthesizing and testing of whole suite of polymer resins and functionalized resins (both self-synthesized and commercial). Besides the in-house capabilities, ORNL has been working with a few major industry companies on evaluating various polymer resins for separations relevant to applications of processing biofuels and bio-products. Examples include weak basic ion-exchange sorbents to selectively remove carbonyl molecules and carboxylic acids from bio-crude oils and aqueous fractions. Lastly, ORNL has strong expertise in highly selective separations based on the high-affinity biosorbents and unique bio-synthetic hybrid sorbents. Besides biomass-based biosorbents, biomolecules can be encapsulated or grafted in immobilization matrices of oxide beads or polymer beads to serve as hybrid biosorbents, which can separate organic compounds and inorganic mineral/metal ions as well. |
| National Laboratory | Oak Ridge National Laboratory (ORNL) |
| Additional Information |
Aimee L. Church, Michael Z. Hu, Suh-Jane Lee, Huamin Wang, Jian Liu. Selective adsorption removal of carbonyl molecular foulants from real fast pyrolysis bio-oils. Biomass and Bioenergy, Volume 136, May 2020, 105522Aimee L. Church, Jianghao Zhang, Yao Yao, Junming Sun, Yong Wang and Hongfei Lin. Renewable Energy Storage via Efficient Reversible Hydrogenation of Piperidine Captured CO2, Green Chemistry 20 (18), 4292-4298 (2018) Aimee L. Church, Andrew Lepore, Jae-Soon Choi, Zhenglong Li, Kim Margrini, Mark Javis, Felipe Polo Garzon, Zili Wu and Michael Z. Hu. Acetic Acid/Propionic Acid Conversion on Metal Doped Molybdenum Carbide Catalyst Beads for Catalytic Hot Gas Filtration. Catalysts 8 (12), 643 (2018) Invention on Sorbent Selective Removal/Reduction of Carbonyls and Carboxylic Acids for Bio-Oil Stabilization and Down-Stream Catalyst Preservation,” Invention Disclosure 201704033, DOE S-138,698, Dec. 14, 2017. Inventors: Michael Z. Hu*, Aimee L. Church. |